ATP Fluorescence Detector
499.00$ USD
As a professional manufacturer of ATP fluorescence detectors, we understand the core requirements of “fast, accurate, and convenient” for sanitary testing. This device can accurately capture microorganisms and biological residues in samples, providing on-site results in 15 seconds without waiting for laboratory reports. Its compact and portable size makes it easy to transport to inspection sites. The high-definition touch screen makes it easy to use, and it also supports 12 hours of continuous testing to batch testing requirements. Whether it’s market supervision and law enforcement, food industry quality control, hospital disinfection inspections, or workshop cleanliness control, it saves time and ensures accurate sanitary testing!
If needed, you can purchase spare swabs:
Click here to purchase spare swabs
Description
| Weight | 1 kg |
| Dimensions | 7.40 × 3.03 × 1.46 inches |
| Accuracy | 1 × 10-18 mol |
| Detection range | 0-999999 RLUs |
| Coliform bacteria | 1-106 cfu |
| Detection time | 15 seconds |
| Detection interference | ±5°C or ±5 RLUs |
| Operating temperature range | 5°C to 40°C |
| Operating humidity range | 20-85°C |
| Detection mode | RLU, coliform bacteria screening |
| Display | 3.5-inch high-precision graphic touch screen |
| Processor | 32-bit high-speed data processing chip |
| USB port | Equipped with a miniUSB port for uploading results to a PC |
| Charging method | Rechargeable lithium battery, no battery replacement required |
| Swab | solid swab, water swab |
Product Features

15-second Rapid Detection
The 15-second rapid testing function eliminates the hours of waiting in traditional laboratories and provides results within 15 seconds of on-site sampling. Whether it’s a regulatory surprise inspection, real-time enterprise quality control, or hospital verification of disinfection effectiveness, it can instantly grasp the hygiene status and quickly identify potential hazards, improving testing efficiency while avoiding delays in health risk management.
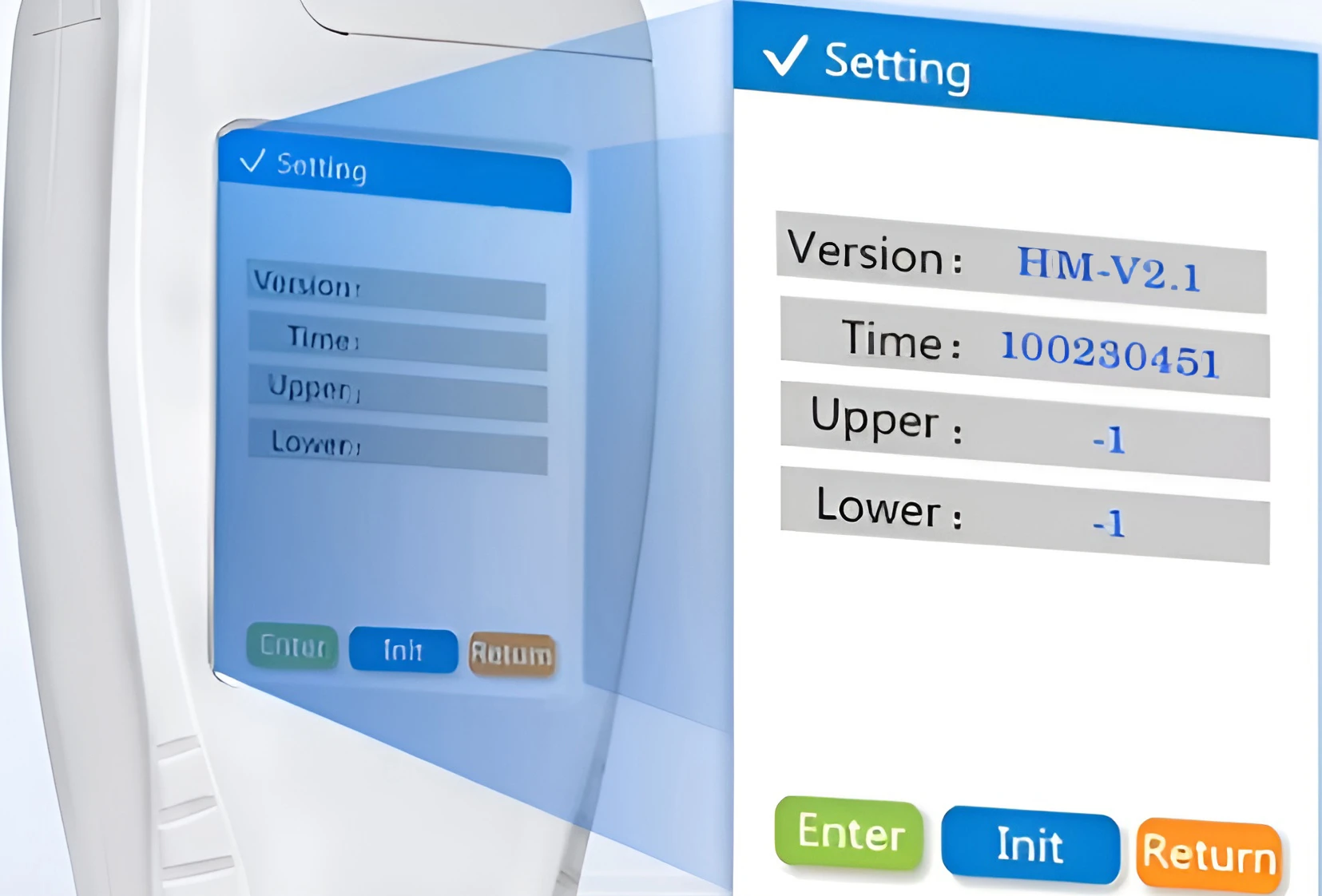
Freely Set Upper and Lower Values
The user-defined upper and lower thresholds allow for custom microbial residue acceptance thresholds based on hygiene standards for various scenarios, such as tableware disinfection and workshop cleanliness. The device provides prompt notifications when test results exceed or fall below the upper or lower limit, meeting the diverse needs of various industries while making hygiene assessments more intuitive and efficient, and reducing human interpretation errors.
Highly Sensitive Imported Photoelectric Sensor
Equipped with a highly sensitive imported photoelectric sensor, it can accurately capture weak fluorescent signals during detection and accurately identify even extremely low ATP levels in samples, effectively avoiding missed detections or misjudgments. At the same time, it can ensure the stability of signal conversion, provide reliable support for subsequent data processing, and further improve the accuracy of test results.
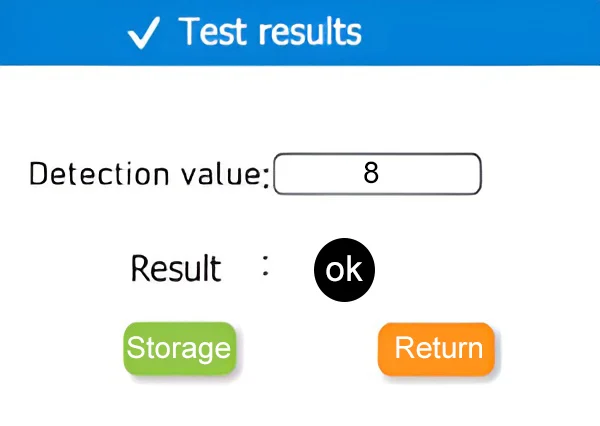
Automatic Result Judgment and Pass Rate Statistics Function
Automatic pass/fail judgment and pass rate statistics eliminate the need for manual comparison and calculation of test data one by one. It can quickly output the result of whether a single sample is qualified or not, and automatically summarize all data and calculate the pass rate. This not only reduces errors in manual recording and calculation, but also significantly saves time in collating results after batch testing, making test result judgment more efficient and statistics more accurate.
12 Hours Continuous Testing
The 12-hour continuous detection capability makes it suitable for batch scenarios such as food workshop random inspections, hospital disinfection inspections, and centralized regulatory inspections. It does not require frequent shutdowns for charging or restarts, and can output results stably, reducing efficiency losses and ensuring the continuous implementation of large-scale health screenings.

3.5-inch High-definition Touch Screen
The 3.5-inch high-definition touchscreen not only clearly displays test parameters, result data, and operational instructions, but also offers a more responsive touch interface. Whether quickly switching test modes, customizing thresholds, or retrieving historical test records, these features can be easily mastered by medical staff, supervisors, and even company personnel, reducing operational barriers and making the testing process more convenient and efficient.

30-second Power-on Self-test
The 30-second power-on self-test function, with the help of a built-in self-calibration light source, quickly verifies the instrument’s core components and system status, preventing distorted test results caused by equipment failure. This ensures that the equipment is in normal working condition before testing, reduces the need for subsequent repeated testing, and effectively improves test reliability.
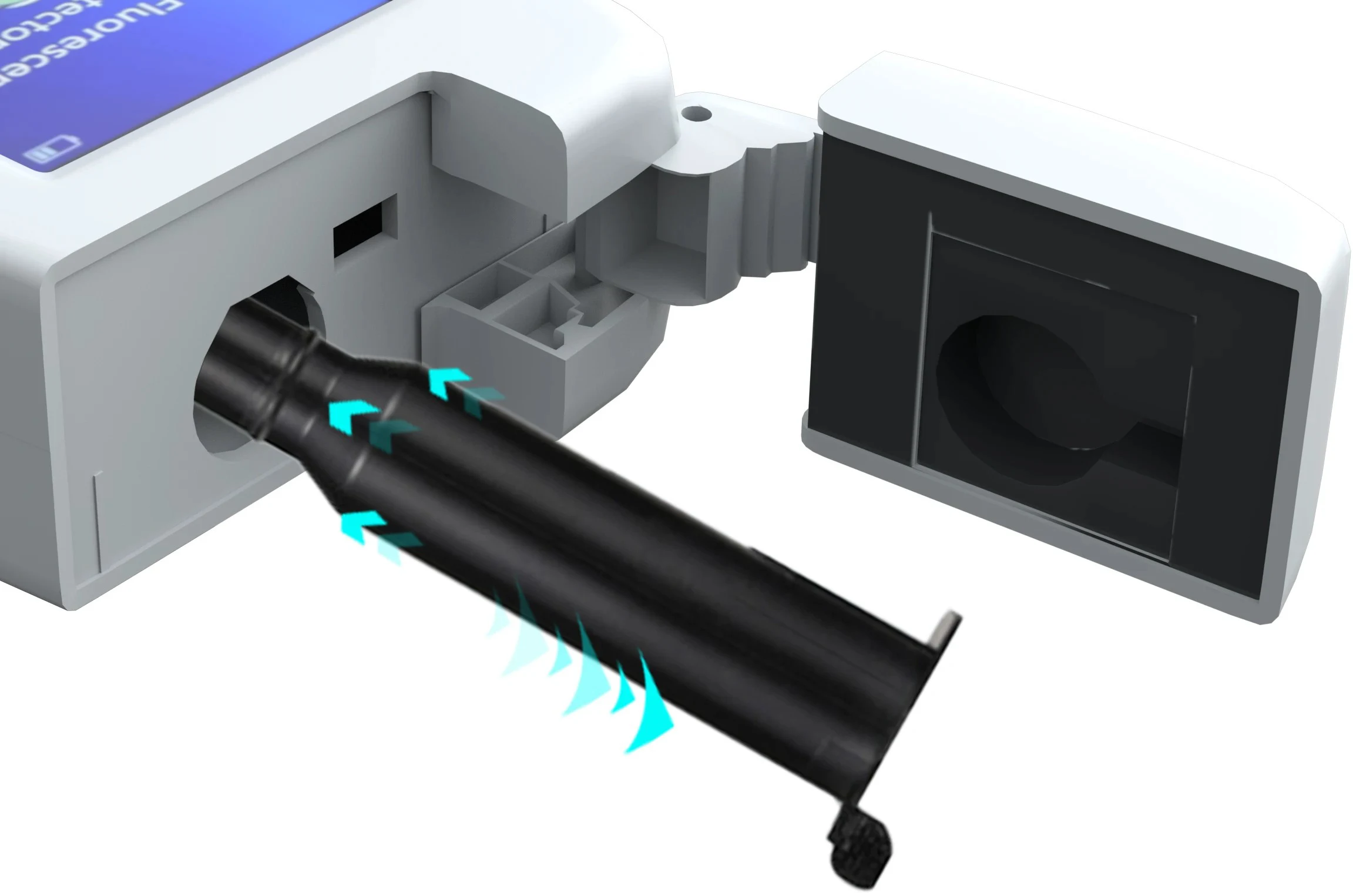
The swab cannula can be pulled out for cleaning
The swab cannula adopts a flexible plug-in design and can be pulled out directly for cleaning. After each test, the inner wall residue can be quickly cleaned to avoid cross-contamination of samples to ensure accurate results. It can also be cleaned regularly for long-term use, which not only improves the user experience but also extends the overall life of the cannula and instrument.

32-bit High-speed Data Processing Chip
Equipped with a 32-bit high-speed data processing chip, it can quickly calculate and analyze fluorescence signal data. It can not only perform rapid detection in 15 seconds, but also efficiently respond to historical record queries, threshold settings and other operations without data lag issues, which not only improves detection efficiency but also ensures smooth equipment operation.

Small Size and Easy to Carry
The ATP fluorescence detector is compact and lightweight, making it easy to hold in one hand or put into a portable tool bag without taking up too much space. It can also easily adapt to scenarios such as portable boxes for outdoor spot checks and narrow workbenches in workshops, without the need for additional large storage areas, greatly improving its portability and flexibility during mobile testing.
Product Detail

Upgrade Swabs
The matching upgraded swab not only has better adsorption capacity and sample release efficiency, can fully collect surface microbial residues to reduce sampling errors, but also has better material compatibility, can adapt to instrument processes and shorten reaction time, which not only improves detection accuracy, but also provides efficient support for batch testing scenarios.

120° Top Cover Opening and Closing Design
The 120-degree top cover opening and closing design provides more ample operating space, allowing users to quickly remove and place swab tubes without having to deliberately control the opening and closing angle. When batch testing is frequently performed, it can reduce hand obstruction and operational obstacles, improving ease of use. It can also avoid component wear caused by too small an opening and closing angle, thereby extending the service life of the top cover.

Mini USB Port
The miniUSB interface allows for convenient connection to computers, chargers, and other devices. It not only allows for quick export of test data for statistical analysis or report retention, but also allows for charging the device at any time, meeting the data transmission and battery life requirements of on-site testing. It’s simple to operate and compatible with common external devices, enhancing flexibility.

USB Flash Drive
A USB flash drive (including computer software) can be directly connected to the device to export data, eliminating the need for additional transfer tools. The exported test data can be quickly organized, analyzed, or used to generate reports using the computer software in the USB flash drive. The software also facilitates subsequent updates and maintenance, simplifying the data processing process and facilitating the archiving and management of test results.

Large-capacity Lithium Battery
The built-in high-performance lithium battery (3AH) provides continuous operation for more than 12 hours and a standby time of more than 300 hours, providing long-lasting battery life without the need for frequent external power supply. Whether it is outdoor water quality sampling, mobile market supervision, or multi-point testing in the workshop, it can ensure the continuous operation of the equipment and avoid interruptions due to insufficient power, effectively improving the flexibility of use and detection efficiency in mobile scenarios.

Aluminum Box
The equipped aluminum box is both sturdy and lightweight. It can not only protect the instrument from collision, extrusion and dust during transportation or carrying, and protect the body and accessories (such as swabs and USB flash drives) intact, but also can neatly store various components to avoid loss or clutter. It is especially suitable for mobile scenarios such as outdoor spot checks and cross-regional supervision, providing guarantees for the portable use and long-term maintenance of the equipment.
Application Scenarios

Water Quality Testing
The scope of application covers drinking water, secondary water supply, industrial water, etc. It can quickly detect microorganisms and biological residues in water bodies and determine whether the water quality is polluted without waiting for laboratory testing. It provides a basis for water quality safety control in a timely manner to ensure water hygiene.

Tableware Surfaces
Applicable to plates, bowls, chopsticks, etc. in restaurants, canteens and other places. It can quickly screen bacterial residues on the surface of tableware, determine whether cleaning and disinfection standards have been met, avoid cross contamination, and provide instant reference for restaurant hygiene control.

Market Supervision
It is suitable for on-site spot checks by supervisors in supermarkets, farmers’ markets, and catering establishments. It can detect the sanitation of food, utensils, and environmental surfaces. The results are available in 15 seconds, improving supervision efficiency and enabling timely detection and disposal of health hazards.

Food Safety
Applicable to food raw materials, semi-finished products in the processing process and the surface of finished products, it can quickly detect microbial residues, timely check risks such as raw material contamination and processing contamination, prevent unqualified food from entering the market, and protect food consumption safety.

Production Workshops
Applicable to key areas such as equipment surfaces, operating tables, and floors in workshops in the food, pharmaceutical, and electronics industries. It monitors environmental cleanliness in real time to prevent microbial contamination from affecting product quality and ensure hygiene compliance in the production process.
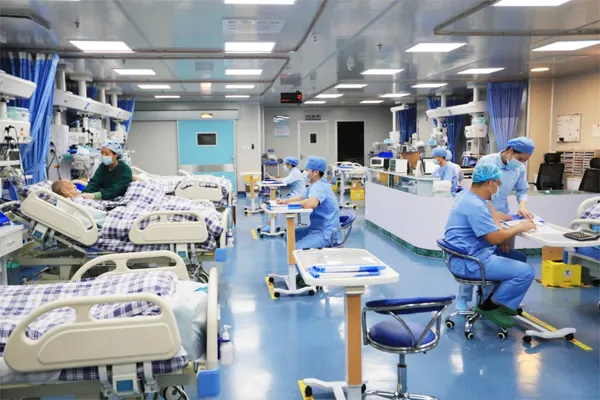
Hospital Disinfection
Applicable to areas such as ward bed units, surgical instruments, diagnostic equipment, corridor handrails, etc., to verify the residual microorganisms after disinfection, determine whether medical and health standards are met, and reduce the risk of cross-infection between doctors and patients.
Other application industries
The instrument is used for microbial monitoring in circulating water and sewage treatment systems, quickly detecting microbial residues, warning of biosludge and water quality deterioration risks, preventing pipe blockage and equipment corrosion, ensuring system stability and reducing the use of chemicals.
The instrument is used for on-site random inspection of food, agricultural products and other inbound and outbound goods, quickly screening for microbial contamination, determining the risk of spoilage or harmful microorganisms, shortening quarantine time, improving customs clearance efficiency, and safeguarding national security.
The instrument is used for microbial testing of clean area environments in production workshops and pharmaceutical raw materials and auxiliary materials. It can quickly verify the effectiveness of cleaning and disinfection, identify potential contamination hazards, avoid excessive microbial counts in pharmaceuticals, ensure compliance with GMP regulations, and reduce production risks.
Industry Standard Thresholds
| Application Scenarios | Eligible scope | Warning value |
| Water Testing – Drinking Water | ≤10 RLU | >10 RLU and ≤50 RLU |
| Tableware surfaces – clean knives, cutting boards, cutlery, etc. | <30 RLU | 30 RLU to 100 RLU |
| Market surveillance – food contact surfaces | ≤30 RLU | 30 RLU to 100 RLU |
| Workshop environment – food processing workshop equipment surface | ≤30 RLU | 30 RLU to 100 RLU |
| Food Safety Testing – Surface of Cooked Meat Products | ≤30 RLU | 30 RLU to 100 RLU |
| Hospital sanitation and disinfection – surgical instruments | ≤150 RLU | 150 RLU to 300 RLU |
Note:In actual application, it should be flexibly adjusted according to specific circumstances, industry norms and relevant standards.
Limit Value Setting
1. Recommended general limit values. If time is of the essence, an ATP hygiene monitoring program should be established immediately. These limit values are determined based on the specific instrumentation and plate counts of the sample being tested.
2. Establishing custom limit values:
① Specimens should be obtained from surfaces that appear clean to the naked eye after each deep cleaning.
② Repeat the test five times and compile the results.
Example 1
| Serial number | Detection value RLU |
| 1 | 26 |
| 2 | 29 |
| 3 | 32 |
| 4 | 19 |
| 5 | 24 |
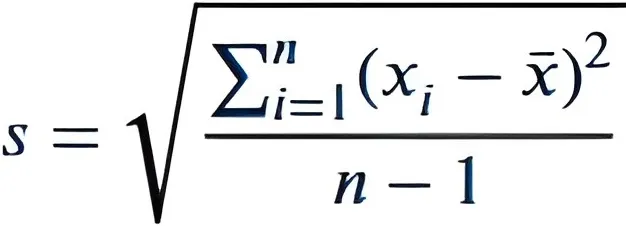
The above are the test results for a specific surface after five cleanings.
① First, calculate the average of these RLU values, which is (26 + 29 + 32 + 19 + 24) / 5 = 26.
② Then calculate the standard deviation (SD) of the data set. The calculation method of standard deviation (SD) is as shown on the left.
③The standard deviation is 4.95 (rounded to approximately 5).
Standard deviation (s.d) is a measure of the dispersion of data distribution, which is used to measure the degree to which data values deviate from the arithmetic mean.Set the limit values as follows:
| Settlement formula | Result |
| Pass < Average | 26 |
| Unqualified ≥ average value + 3 times the standard value deviation | 26+5×3 |
| Be wary of deviations ≥ average value < 3 times the standard value | 26-41 |
Note: If the user already has a control point total colony count standard plate count standard, the ATP test results can be used as a reference. However, please note that ATP comes from microorganisms or organic residues, so it can only be said that ATP levels are closely correlated with the number of microorganisms. There is not a complete one-to-one correspondence between ATP testing and plate count results, but there is a close correlation.
Device Operation Page

Front Page
Press the button on the left side of the instrument, turn on the instrument and calibrate for 30 seconds before the home page appears.
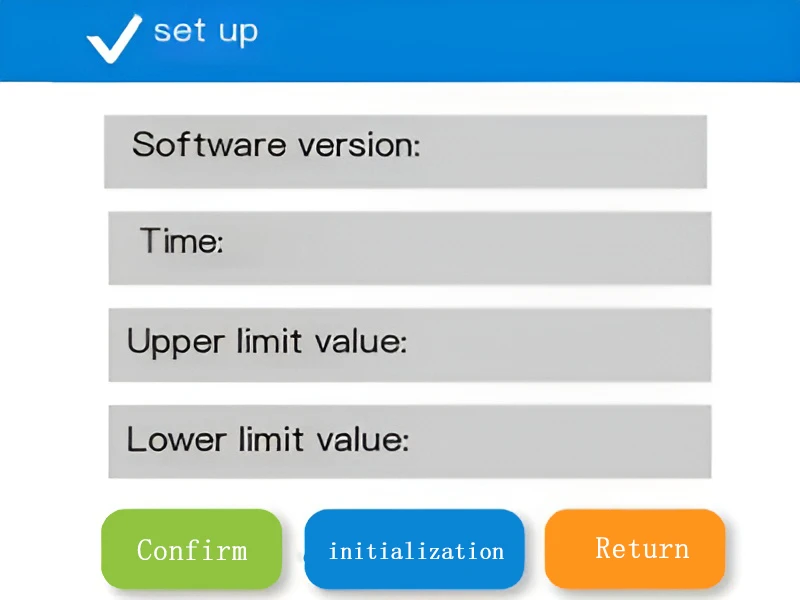
Set up
The setting page of the ATP fluorescence detector supports the adjustment of upper and lower limits, time and other parameters. It can adapt to the detection standards of different scenarios, meet personalized needs, make the result judgment more in line with reality, and improve the flexibility and applicability of the detection.
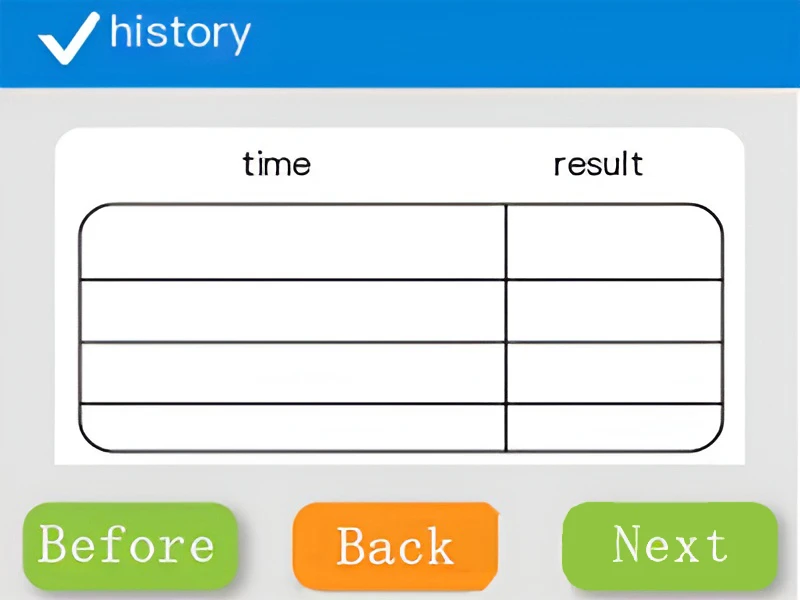
Historical Test Data
The historical detection record function of the ATP fluorescence detector can easily trace past detection data, facilitate comparison of changes in hygiene conditions at different time periods, help analyze the effects of cleaning and disinfection, and troubleshoot potential problems. At the same time, it provides a reliable historical basis for hygiene management and supervision, and improves work efficiency.
Detection Principle of ATP Fluorescence Detector
The core mechanism of ATP fluorescence detection is the luciferase-luciferin system, based on the principle of firefly luminescence. All living biological cells (including microorganisms) and residual organic matter contain the energy molecule ATP. During the test, ATP in the sample reacts with luciferase and luciferin in specialized reagents under specific conditions, releasing energy and stimulating fluorescence. The intensity of the light signal is proportional to the ATP content. The instrument converts the light signal into relative light units (RLUs), which directly reflect the amount of ATP in the sample. Because ATP content is positively correlated with the number of microorganisms and residual organic matter, the RLU value can be used to quickly and indirectly determine the degree of contamination. Compared to traditional microbial culture methods, this test eliminates the need to wait for bacterial proliferation and provides instant results, enabling rapid assessment of sanitary conditions.
Swabs
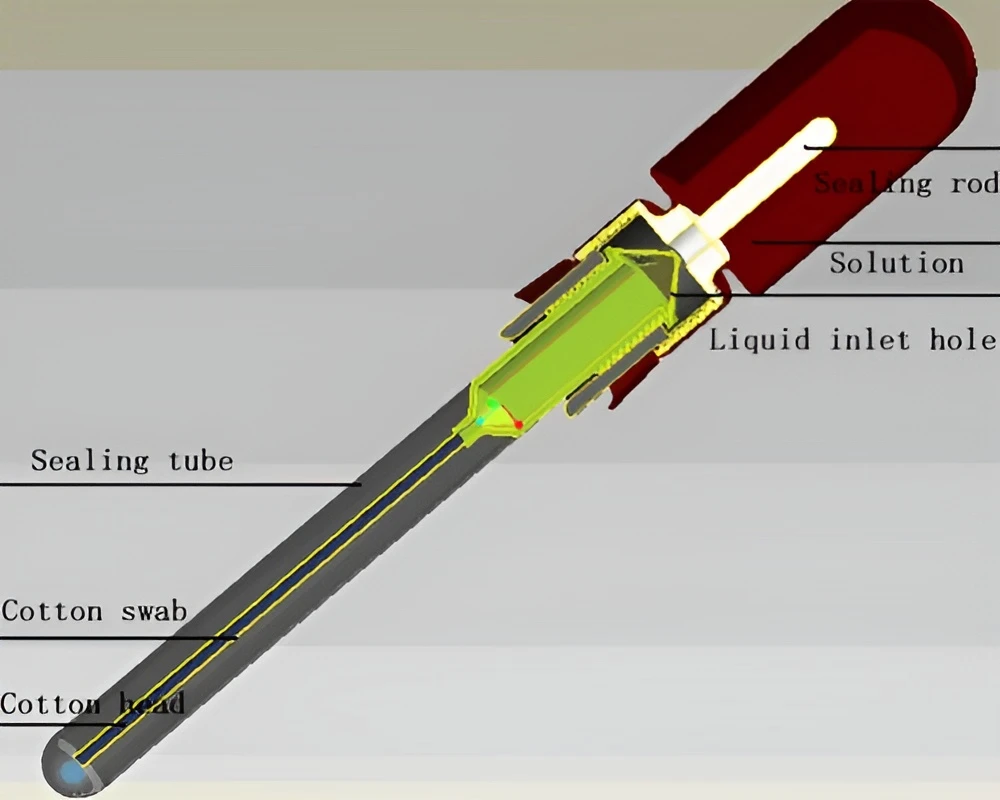
Moistened All-in-one Collection Swab
The ATP bioluminescence detection swab is a dedicated integrated device that needs to be used with an ATP fluorescence detector. It integrates sample collection, reaction and rapid detection functions. It uses advanced liquid stable reagents, which is not only more convenient to use, but also can bring more accurate test results, and the luminescence signal lasts longer and has better repeatability. Structurally, the swab is mainly composed of three parts: a sealing cap, a sealing tube and a cotton swab.There are two types of test swabs: solid surface swabs and water sample swabs.
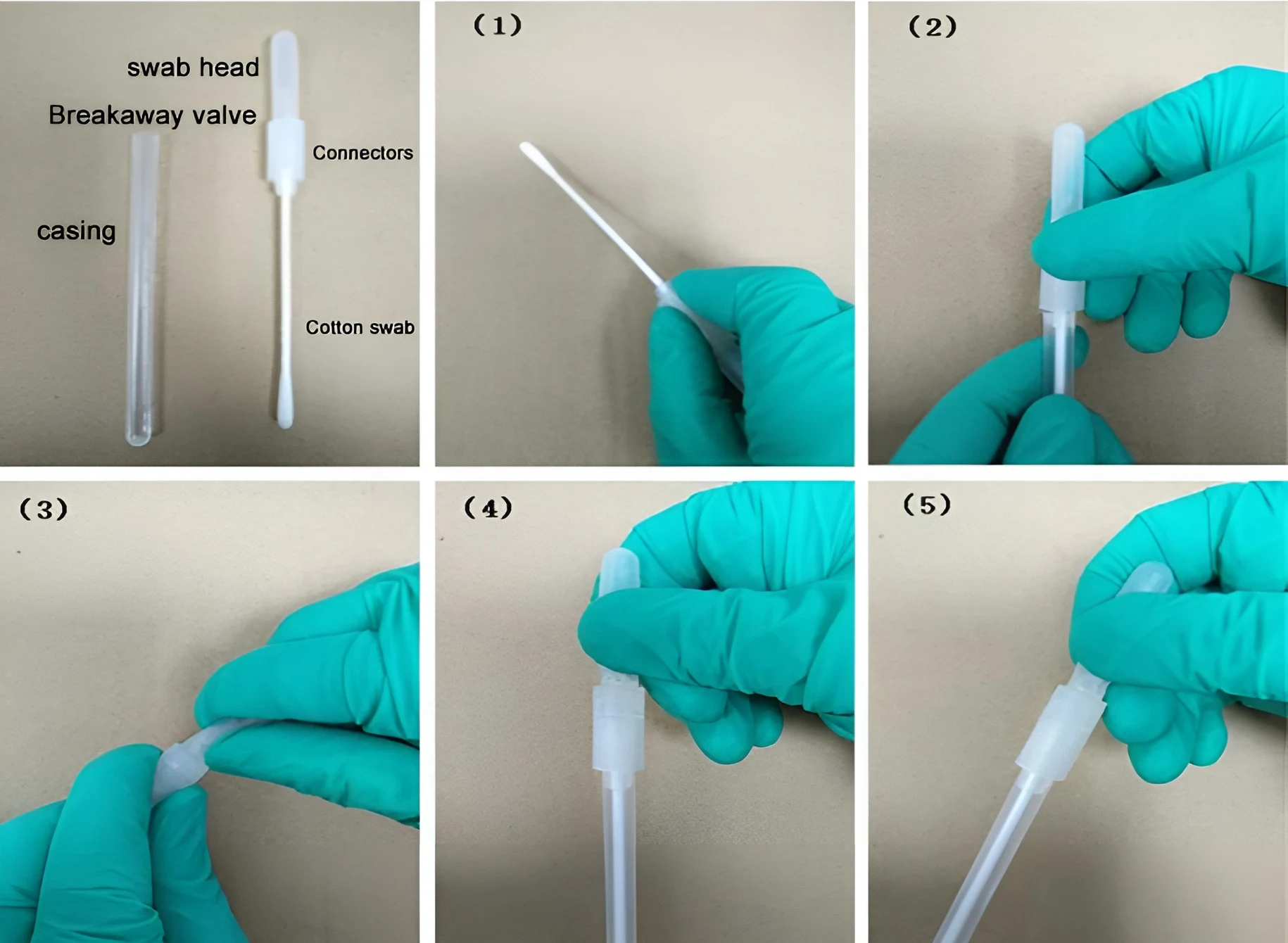
Solid Surface Swabs
1.Remove the swab from the refrigerator and let it cool to room temperature for 20-30 minutes, or warm the top of the swab capsule with your palm for 5 minutes to activate it.
2. Remove the swab from the cannula, pinch the connector firmly, and thoroughly wipe the surface at a 45° angle. (Figure 1)
3.Insert the collector back into the cannula and tighten. (Figure 2)
4. Pinch the connector with one hand and the top of the capsule with the other, bending it back and forth until the valve snaps off. (Figure 3)
5. Squeeze the swab tip three times to remove all liquid. (Figure 4)
6. Hold the swab tip firmly and shake it downward five times, left and right. Immediately insert it into the instrument for testing. (Figure 5)
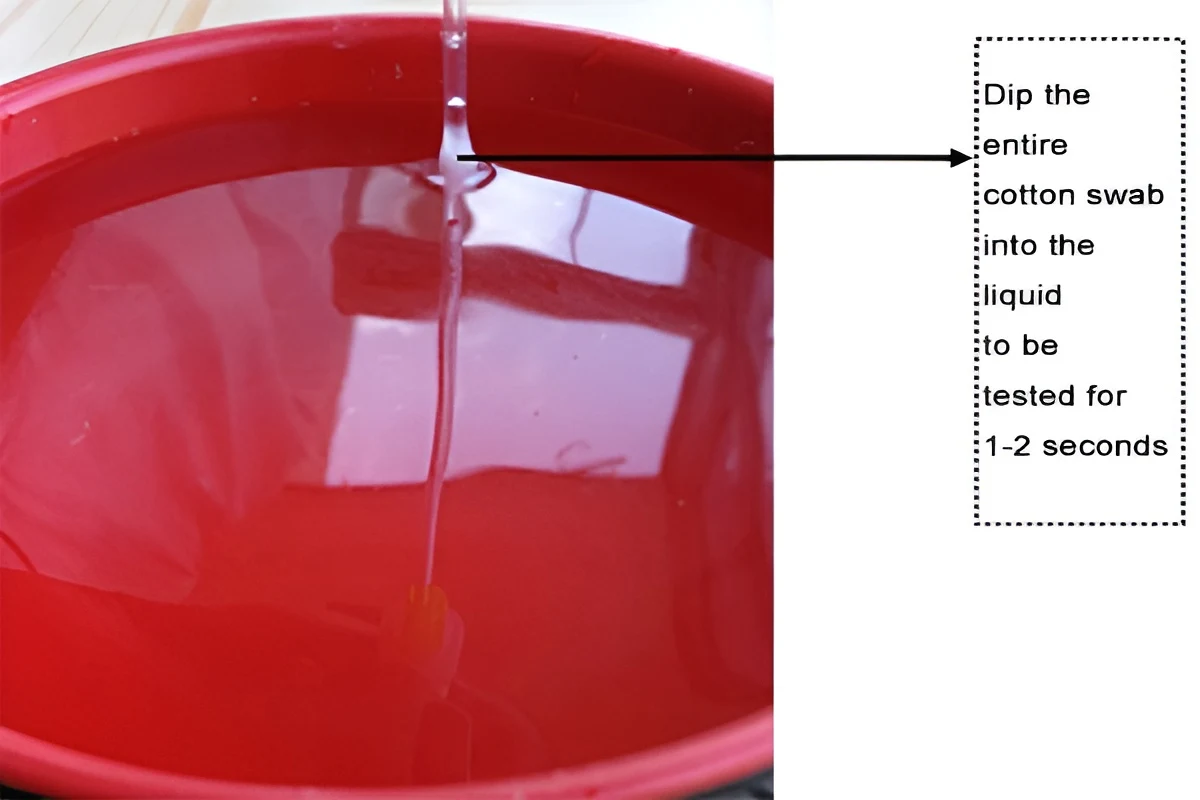
Water Swab
1. Allow the refrigerated or frozen swab to rest at room temperature for approximately 25 minutes (refer to surface swab procedures).
2. Gently unscrew the lower tube, taking care not to allow the swab to touch other objects and to avoid prolonged exposure to air.
3. Completely immerse the swab in the test solution for 1-2 seconds, then quickly remove it and insert the sealing tube.
4. Break off the sealing rod and squeeze to allow the test solution to flow to the cotton tip. Shake the bottom of the swab for approximately 5 seconds.
5. Quickly place the swab in the ATP fluorescence detector, press the test button, and read the result after 15 seconds.
ATP Bacteria Detection Steps
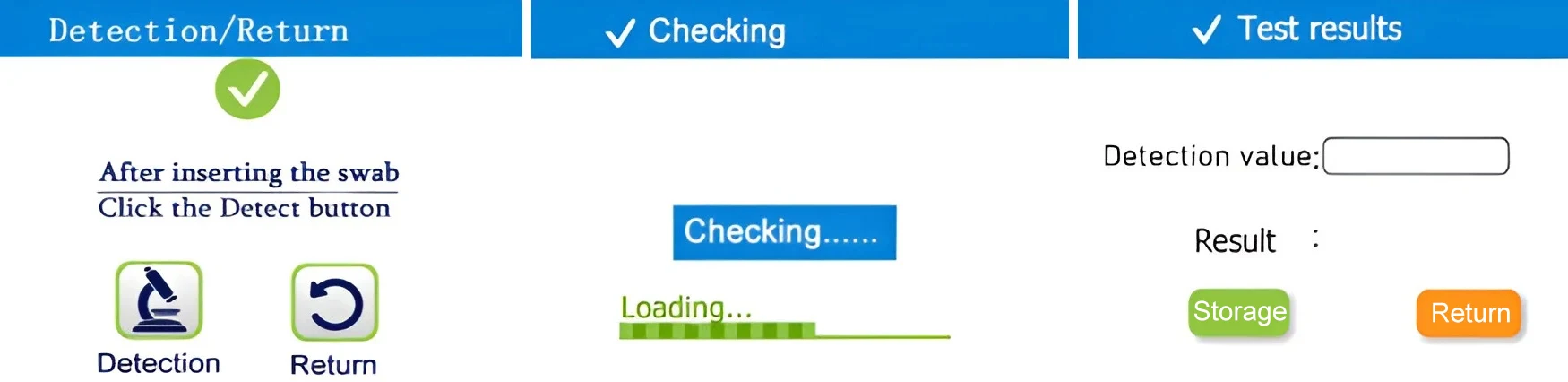
1. Turn on the device and click “Detection” ,The instrument will prompt “Insert swab” to enter the test interface.
2. Different swabs require different methods of use; proceed accordingly.
3. Click “Detection” Test results will be available in 15 seconds. (Manually save results.)
Test results
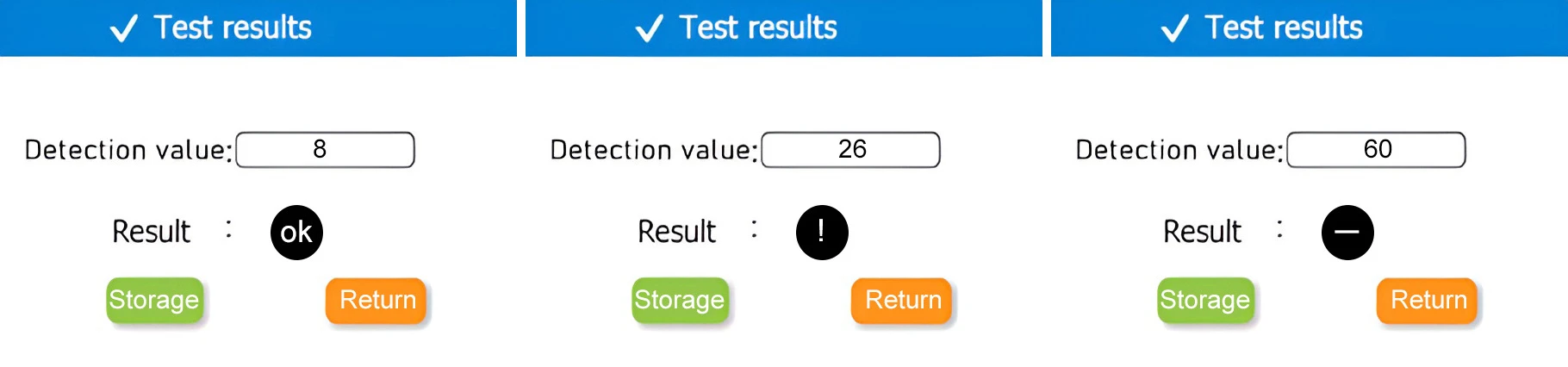
Note:Take “Water Testing – Drinking Water” as an example.Qualified line ≤ 10RLU, warning line > 10RLU and ≤ 50RLU.
| notation | notation interpretation |
| “OK” | Detection data ≤ lower limit value |
| “!” | Detection data≥lower limit value ≤ upper limit value |
| “-“ | Detection data>upper limit value |
How to upload data to PC?
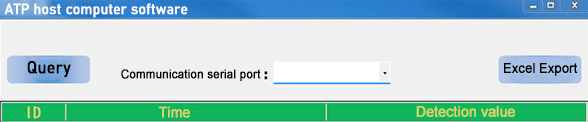
1. Install the host computer software: copy the host computer software installer in the U disk to the computer, double-click the installation file, and follow the prompts to complete the software installation. If the installer cannot run automatically, please open the U disk folder and run the installer manually.
2. Connect the data cable: Use the data cable that comes with the instrument, insert one end into the miniUSB interface of the ATP fluorescence detector, and connect the other end to the USB port of the computer.
3. Open the host computer software: From the “Start” menu or desktop shortcut of the computer, find and open the installed ATP fluorescence detector host computer software.
5. Select the communication serial port: Click the inverted triangle on the right side of the communication serial port to select the serial port connected to the device.
6. Click Query: Query the historical data of the device.
7. Click “Excel Export” to export data: the export format is “Excel” format.
Packing List

ATP Fluorescence Detector

Swab

Aluminum Box

USB Flash Drive

Data Cable

Charging Head
Accessories Available for Purchase

ATP Special Swab
The ATP fluorescence detection swab is a dedicated companion tool for ATP fluorescence detectors, integrating sample collection, ATP cleavage, and reaction in one device, eliminating the need for additional reagent preparation. Its advanced liquid-based stable reagent is ready for use and prevents uneven dissolution, minimizing impurity interference, improving detection accuracy, and ensuring data reproducibility. Available swabs are compatible with both solid and water samples, facilitating rapid health screening in a variety of scenarios.
Go to purchase!phone: +1 402 915 1622
Email:hi@hygienaatpmeter.com
Address: 12 E Twohig Ave #200-F12, San Angelo, TX 76903, USA
Reviews (0)
Be the first to review “ATP Fluorescence Detector” Cancel reply
You must be logged in to post a review.














Reviews
There are no reviews yet.